Blue Arbor Technologies Inc. has achieved a significant milestone with its RESTORE Neuromuscular Interface System, designed for individuals with upper limb loss. The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to this innovative system.
In addition, the RESTORE System has been accepted into the FDA’s Total Product Life Cycle (TPLC) Advisory Program (TAP) Pilot. This recognition highlights the system’s potential to enhance prosthetic technology by integrating the peripheral nervous system with robotic prosthetics, aiming to restore naturalistic hand and arm function.
Aviram Giladi, M.D., research director at The Curtis National Hand Center, praised the system’s transformative potential, citing early studies that indicate improved functionality for individuals with prosthetic upper limbs.
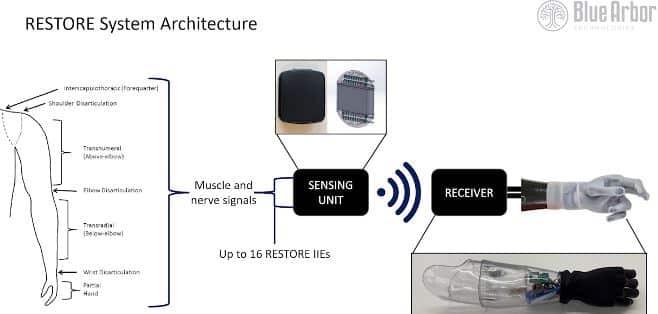
The RESTORE System offers a direct connection to the patient’s residual muscles and peripheral nerves, enabling reliable, voluntary movement control signals. This approach surpasses current interface technologies, enhancing functionality and usability for users.
With an increasing number of Americans living with limb loss, the need for advanced prosthetic solutions is urgent. Blue Arbor Technologies aims to address this need by developing innovative solutions that improve prosthetic control and integration into daily life.
Paul Cederna, M.D., president of Blue Arbor Technologies, expressed the company’s commitment to improving the lives of individuals with limb loss. The Breakthrough Designation and TAP enrollment signify significant progress towards FDA market clearance, bringing the RESTORE System closer to benefiting those in need.
The FDA Breakthrough Devices Program and TAP Pilot aim to expedite the development and commercialization of innovative medical devices.